Abstract
Review of the periodic table and existing research on isotopes of the various elements of the periodic table is conducted and the attempt is made here to visualize the process of element formation.
The role of Neutron in element formation is investigated and found to be vital for existence of elements . In the new light, the new model of nucleus is proposed which explains the stability of the nuclei and reason for multiple stable isotopes of elements.
Review of the Periodic Table
The inspection of the periodic table of elements reveal an interesting fact that for all elements other than Hydrogen, for element to be stable, number of Neutrons are always greater or equal, to the number of Protons in the nucleus. The periodic table is analyzed with respect to the abundance in nature for the elements.
The fusion of Hydrogen to produce Helium can be envisioned as follows:
1H + 1H + 1H + 1H → 2H + 2N + 2e+ (1)
where 2e+ later annihilating to produce additional gamma radiation.
Since tremendous force is needed to keep four protons together during the formation of helium and emit 2 positrons, it is unlikely. The mass of neutron is more than the mass of proton and this fact alone negates the likeliness of the above scenario.
- Neutrons are the building blocks for elements in nature.
- In the elements , other than hydrogen, neutron and proton form a pair (np) and keeps distinct identity.
- Excess neutrons stay at the center of the nuclei but keeps their distinct identity.
- Abundance of isotope is close to 100% when excess neutrons are odd count.
- If the excess neutron count is even, the isotope is radio active.
- Majority elements have only one Isotope.
- Only Potassium has three isotopes, the one with 2 excess neutron is radio active with 0.001 relative abundance.
- Elements He, C, O, Ne, Mg, Si, S are close to one in abundance with no excess neutron and maximum three isotopes.
- Element Be has one excess neutron and one isotope, 100% abundance.
- All other higher elements require four or more excess neutrons for the element to be abundant in nature and has up to 10 stable isotopes.
- Element Ca is exception with 0 excess neutron and 97% abundance. However it is unstable with >E+21 a Half life.
The nature prefers, for more complex nucleus, more neutrons than protons and creates a delicate balance to form a stable nuclei.
This balance of forces is so critical that in case of element F (the stable isotope is with 9 proton and 10 neutrons) The isotope 18F with 9 Protons and 9 Neutrons, with in 20 minutes decays and forms 18O which is stable with 8 protons and 10 Neutrons and gives up a e+ positron to convert proton to neutron and e+ and e- reaction produces Gama radiation.
Equation 18F → 18O + e+
Thus the existence of Neutron is vital to the existence of the universe itself, because without neutron, elements may not have been possible and hence the intelligence as we know today.
Structure of Neutron
The fact that Gravitational binding forces (Fg) of masses in the nucleus needs to be more than the destructive electromagnetic forces (Fem) created by the electrically positive environment of the nucleus, (Fem < Fg) excess neutrons are required in the nuclei. However, even at the center of the nucleus neutrons keep their distinct identity, rather than lumping together to form one heavy neutron. Therefore, neutron can not be just a fuzzy mass but a well defined structure able to hold induced charges with precise demarcation of boundary with an insulating layer, more like a free standing capacitor. Which suggests that at least three particles with two sets of characteristics are required to create a neutron. For our model here, that has to be two particles capable of holding charges and one particle which provides insulation between these particles, similar to that of dielectric layer in the capacitor. Thus in this postulated model the neutron looks and behaves neutral for all practical purposes. However, within nuclei keeps separate and distinct identity and does not combine with other neutrons to form a one central heavy body.
The proposal here defers from the model independent analysis predicting the structure having negative charge on the surface, Positive charge in the next lower level and than neutral mass. My model suggests that neutron is a free standing charged capacitor. This helps in keeping the distance between excess neutron in the nuclei. Otherwise there is nothing stopping these neutrons from lumping together to form a mini neutron star with in the nuclei. This model of Neutron is justified from the known fact that Neutron is heavier than Proton.
For elements other than Hydrogen, the nuclei becomes a chaotic environment. For Example, in case of
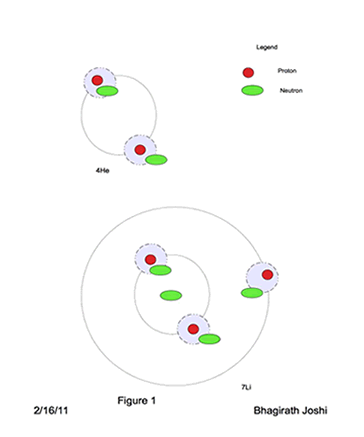
4He, two protons require their space and wants to be separate from each other as far as possible, and two neutrons are caught between the two protons as shown in figure 1. From the reference frame of protons it is envisioned that Neutrons spin on its axis at very high rotational speed. One clockwise and other anticlockwise.
Model of nuclei
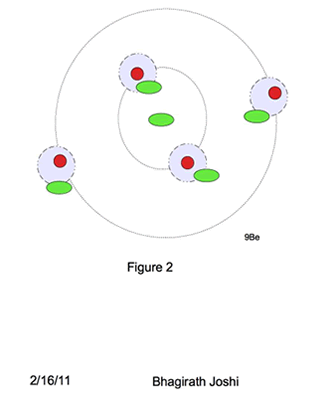
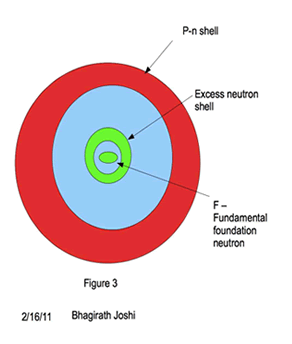
A new model of nuclei is proposed as shown in the figure 3. A spherical shell of excess neutrons with one neutron at the center of the shell surrounded by paired proton neutron (pn) shell.
For the stable nuclei system the electromagnetic forces needs to be balanced and gravitational forces maximized. From the example of 4He above, it is envisioned that elements are built, in this model, by Heavy Hydrogen nuclei (pn) as a building block and just enough excess neutrons to provide needed gravitational force for stability.
Energy levels (orbitals) in pn outer shell follows the similar shell structure of electrons, with K,L,M,N,O being primary shell and s, p, d, f, g sub shells with similar total charge particle capacity. However for excess neutron shell it differs, where a single neutron stays at the center of the nuclei when in excess. The energy level for that central neutron in this model is called “Foundation neutron” (Fn).
The Table 4 shows the placement of neutrons in each shell.
Nuclei Stability Analysis using above model
For Odd atomic number nuclei:
In the ‘p-n shell’ the outermost pair has no symmetry, however the excess neutron shell is symmetrical for all odd atomic number elements giving the stability and abundance. Referring to table 2 and table 4 , isotopes, with one excess neutron, has relative abundance in nature of 1 or close to 1, The excess neutron takes the place of Fn. The isotopes with 2 excess neutrons are all radio active which can be attributed to the asymmetry of neutron in unfilled K shell, which can hold up to 2 neutrons. When the K shell is completely filled as in the case of 41K, the nuclei is stable. Table 2 outlines isotopes and abundance for majority of elements, all follow the same principle and model fits perfectly with the exception of 14N with atomic number 7. Iodine has 21 excess neutrons, as we see in table 4, the outer most excess neutron shell is symmetrical, making it stable. All other elements follows.
For Even atomic number nuclei:
The stability is obtained from the pn shell. However, excess neutron shell becomes asymmetric and depending on which sub shell, there is a room for additional excess neutrons and hence exhibits many stable isotopes with relative abundance. Theoretical probability calculation may prove this fact.
Instability and radio activity in heavy elements:
The neutron proton (np) pair in nuclei has capability to grow indefinitely. However as the heavier elements are built the inner excess neutron shell is large enough to interact with lower np shells, thus giving instability to the nuclei. e.g. for 238U there are 148 total neutrons and 54 excess neutrons. A nuclei with 111 excess neutron will completely fill up the O shell. All elements after 209Bi are radio active, this shows that interaction of neutrons form excess neutron shell(inner shell) with pn shell is destructive when the N shell is half filled. Thus there is a upper limit to the size of the inner excess neutron shell and this may be the reason for non existence of super heavy stable elements in nature.
Conclusion
This proposed model of nuclei, explains the stability and relative abundance of the nuclei. The nuclei(except for H and He) in this model is a shell with in a shell, where an innermost shell structure is formed with excess neutrons with one Fundamental foundation neutron (Fn) and the rest arranged in shell structure. Protons, form pair with neutron and form shell enclosing this excess neutron shell as shown in figure 3. The stability of the nuclei is a direct result of symmetrical placement of neutrons in the outer most shell of excess neutron shell. The same shell is responsible for many stable isotopes in even atomic number nuclei. The additional isotopes are possible if the outer shell of excess neutron shell holds odd number of neutrons. The nature of neutron is postulated here, the proof of which depends on future experiments.
References
Numerous Scientists’ and scholars’ exhausting work in development of periodic table, investigation of Isotopes is utilized and due credits are given. The list is too large to print here, but due credits are mentally given to all scientists.

@Corinne:
Your comment was past due !
Thank you,
Norma