by
A.V. Simakin and G.A. Shafeev
Wave Research Center of A.M. Prokhorov General Physics Institute of the Russian Academy of Sciences, 38, Vavilov street, 119991 Moscow Russian Federation.
Abstract:
Laser exposure of suspension of either gold or palladium nanoparticles in aqueous solutions of UO2Cl2 of natural isotope abundance was experimentally studied. Picosecond Nd:YAG lasers at peak power of 10^11 -10^13 W/cm^2 at the wavelength of 1.06 – 0.355 µm were used as well as a visible-range Cu vapor laser at peak power of 10^10 W/cm^2. The composition of colloidal solutions before and after laser exposure was analyzed using atomic absorption and gamma spectroscopy in 0.06 – 1 MeV range of photon energy. A real-time gamma-spectroscopy was used to characterize the kinetics of nuclear reactions during laser exposure. It was found that laser exposure initiated nuclear reactions involving both 238U and 235U nuclei via different channels in H2O and D2O. The influence of saturation of both the liquid and nanoparticles by gaseous H2 and D2 on the kinetics of nuclear transformations was found. Possible mechanisms of observed processes are discussed.
PACS numbers: 42.62.–b; 61.46.+w; 78.66.–w
*Corresponding author, e-mail shafeev@kapella.gpi.ru
Introduction:
Modern lasers allow excitation of nuclear energy levels via generation of high-energy particles that appear during the interaction of laser radiation with plasma produced on a solid target. Successful excitation of nuclear levels has been reported for some isotopes of Hg and Ta under exposure of a target in vacuum to a femtosecond laser radiation. [1,2]. Emission of gamma-photons from a Ta target exposed in vacuum to peak intensity of 10^18 W/cm^2 in a femtosecond range of pulse duration results has been reported recently [3]. The average energy of these photons is about few MeV. Picosecond laser plasma is also a source of high-energy particles whose energy is sufficient for excitation of energy levels of nuclei in the exposed target [4].
Another possibility for laser excitation of nuclear energy levels consists in laser exposure of nanoparticles suspended in a liquid (colloidal solution). This scheme allows laser initiation of nuclear reactions, e.g., transmutation of 196Hg into 197Au [5, 6] via laser exposure of Hg nano-drops in heavy water D2O. It is believed that thermal neutrons needed for this transmutation are released from Deuterium though the mechanism of this release remained unknown. The possibility to induce nuclear reactions at relatively low peak intensity of laser radiation was attributed to the local field enhancement in the vicinity of metallic nanoparticles by a factor of 10^4-10^5. This may provide effective peak intensity in the liquid of about 10^17 W/cm^2, which is already comparable with those used for exposure of solid targets in vacuum.
It is of interest to use the same approach for initiation of nuclear reactions in nanoparticles of unstable elements, such as 238U or 232Th. However, these elements are chemically reactive and would react with aqueous environment during the laser synthesis. The solution of this problem consists in using NPs of noble metals, e.g., Au, to provide the constant level of absorption in the liquid, while unstable elements can be presented in the solution as aqua-ions [7].
The aim of this work is the experimental study of possibility of laser initiation of nuclear reaction in aqueous solutions of a Uranium salt under absorption of laser radiation by Au nanoparticles. The most known Uranium isotopes are 238U and 235U. They undergo the sequence of α- and β-decays as follows:

Raw Uranium contains 0.7% of another isotope 235U that originates from 235Np. Natural decay time of both Uranium isotopes is very long (4.5×10^9 and 7×10^8 years, respectively). One may expect that thermal neutrons generated through laser exposure of Au NPs in aqueous solutions should alter the equilibrium concentration of all elements that belong to U branching.
Experimental:
Au nanoparticles (NPs) were synthesized by ablation of a bulk gold target either in H2O or D2O with the help of a Nd:YAG laser with pulse duration of 70 ns at wavelength of 1.06 µm. The details of the synthesis can be found elsewhere [8, 9]. The resulting average size of Au NPs as determined by Transmission Electron Microscopy lies between 10 and 20 nm. The Uranium salt UO2Cl2 of natural isotope composition was then dissolved in the colloidal solution, and the solution was divided into two parts, one of them considered as the initial solution. The second part of the solution was exposed to laser radiation. The exposure was carried out either of the as-obtained solution or under continuous purge of H2 or D2 for H2O and D2O, respectively. The gases were obtained by electrolysis of corresponding liquids, either H2O or D2O and were supplied to the solution at atmospheric pressure.
Three laser sources were used for exposure on Au NPs in the aqueous solutions of the Uranium salt. These were a Nd:YAG laser, pulse duration of 150 ps, wavelength of either 1.06 or 0.355 µm, energy per pulse of 100 at 1.06 and 20 mJ at 0.355 µm, repetition rate of 10 Hz, peak power of 10^13 W/cm^2, a Nd:YAG laser, pulse duration of 350 ps, wavelength of 1.06 µm, energy/pulse of 350 µJ, repetition rate of 300 Hz, peak power of 10^11 W/cm^2, and a Cu vapor laser, pulse duration of 10 ns, wavelength of 510/578 nm, energy/pulse of 100 µJ, repetition rate of 15 kHz, peak power of 10^10 W/cm^2.
Gamma-emission from samples before and after laser exposure was characterized using a semiconductor γ-spectrometer Ortec-65195-P. This provided the analysis of sample specific activity in γ-photons from 0.06 до 1.5 MeV in Becquerel per ml. Real-time acquisition of γ-spectra of the solutions during laser exposure was achieved with the help of a portable scintillator γ-spectrometer. In the latter case the cell with the solution was fixed just on the spectrometer itself, which guaranteed the constant geometry of measurements under natural background of γ-radiation. The acquisition time was sufficiently long to provide the accuracy of measurements better than 3% in the channel with maximal number of counts indicated by the spectrometer.
Results and discussion:
Exposure of Au NPs in aqueous solutions of UO2Cl2 either in H2O or in D2O leads to significant modifications of the activity of all elements of U branching. The result of the laser exposure depends on the kind of water used in the experiment. Exposure in D2O results in the decrease of the activity of both Uranium isotopes at laser peak power of 10^10 -10^11 W/cm^2. Activity is linearly related to the quantity of the corresponding isotopes therefore, one may conclude that laser exposure of Au NPs in presence of aqua-ions of UO2^-2 leads to the accelerated decay of 238U.
In case of laser exposure of Au NPs in H2O with UO2Cl2 the result is the opposite. 238U is not gamma-active, and the modifications of its concentration can be inferred from the activity of its daughter nuclides, 234Th and 234Pa. The activity of 234Th and 234Pam, as well as 234Pa increases after laser exposure (see Fig. 1, a). Note that these elements are daughter ones for 238U. In Fig. 1, a one can see that the activity (concentration) of 231Th also increases after laser exposure. The parent of this element is 235U, and the increase of 231Th signifies its accelerated decomposition. However, the concentration of 235U increases after laser exposure as it is shown in Fig. 1, b.
It is pertinent to note that no measurable changes of the activity of nuclides of U branching were detected under exposure of the colloidal solutions of Au NPs in either H2O or D2O with UO2Cl2 with radiation of a femtosecond radiation of a Ti:sapphire laser at peak power of 10^13 W/cm^2 at wavelength of 800 nm.
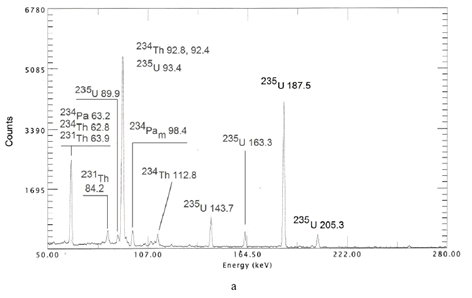


Fig. 1. Gamma-spectrum of the initial solution of UO2Cl2 in H2O with Au NPs (a). Gamma-spectrum of elements of 238U branching before (black) and after laser exposure (red) of the colloidal solution of Au NPs in H2O with UO2Cl2 (b). Gamma-spectrum of 235U before (black) and after (red) laser exposure of the colloidal solution of Au NPs in H2O with UO2Cl2 (c). Cu vapor laser 4 hours of exposure, peak power of 10^10 W/cm^2, repetition rate of 15 kHz.
Real-time γ-spectra of the samples are presented in the Fig. 2.
 Fig. 2. Differential spectra of the samples of Au NPs exposed to 350 ps laser radiation in H2O with purged H2 (a) and in D2O with purged D2 (b). Initial spectra of the same sample are subtracted in each case.
Fig. 2. Differential spectra of the samples of Au NPs exposed to 350 ps laser radiation in H2O with purged H2 (a) and in D2O with purged D2 (b). Initial spectra of the same sample are subtracted in each case.
One can see that the samples are characterized by γ-emission of the nuclides belonging to 238U branching as well as by that of 235U. However, different peaks of these nuclides are active under laser exposure in H2O and D2O at otherwise equal conditions.
The tendency changes at higher peak power of the laser radiation. Namely, at the peak power of order of 10^13 W/cm^2 in 150 ps pulses the activity of both U isotopes increases after laser exposure of the colloidal solution of Au NPs in D2O. This is illustrated in Fig.3.
 Fig. 3. Gamma-spectrum of the sample of UO2Cl2 in D2O exposed to the first harmonics of a Nd:YAG laser, pulse duration of 150 ps, 1 hour of exposure at 10 Hz.
Fig. 3. Gamma-spectrum of the sample of UO2Cl2 in D2O exposed to the first harmonics of a Nd:YAG laser, pulse duration of 150 ps, 1 hour of exposure at 10 Hz.
The activity of both U isotopes increases after the laser exposure of Au NPs in D2O, as it can be concluded from the increase of activity of corresponding daughter nuclides. The kinetics of the nuclear transformations is also sensitive to the laser wavelength. The dependence of the activity of several nuclides of U branching on the concentration of UO2Cl2 is presented in the Fig.4.
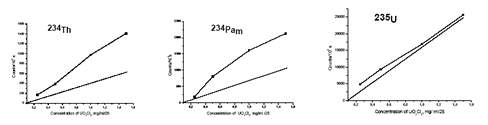
Fig. 4. Dependence of activity of 234Th, 234Pam, and 235U of the same probes exposed to the 3rd harmonics (0.355 µm) of a 150 ps Nd:YAG laser in H2O for 1 hour at 10 Hz repetition rate on the concentration of Uranium salt. Straight lines represent the activity of the same nuclides in the initial solution.
In this case the laser action is characterized by high selectivity. Indeed, the decay of 238U is noticeably accelerated by laser exposure of Au NPs along the branch 238U → 234Th→ 234Pam→ 234Pa, and the activity of 234Th in the laser-exposed sample is twice higher than in the initial sample. On the contrary, the activity (and related to it concentration) of 235U remains almost constant in the same probes.
Different reaction pathways observed under exposure in H2O and D2O imply different interaction of these compounds with Au NPs. This interaction is not related to chemical one since chemical properties of these two waters are the same. Indeed, NPs are molten during their synthesis by laser ablation and ionized during laser exposure. The emission of atomic Au has been detected under exposure of Au NPs in water at laser peak power of 10^11 W/cm^2 at 1.06 mm wavelength. The upper electronic level of this emission is 5 eV, which is comparable with the energy of dissociation of water molecules (13.6 eV) [9]. Accordingly, the water vapor around the NPs is partially dissociated. Molecular gases H2/D2 dissolve in the metal while the solubility of O is much lower than that of H/D due to larger size. This process is very efficient in view of high specific surface of Au NPs used in this work since their surface is as high as 10 m^2 per 1 ml of colloidal solution. Saturation of the liquid with H2/D2 increases the quantity of these gases in Au NPs. If the solidification rate of NPs is sufficiently high, then the dissolved gases remain inside the NPs. Saturation of the liquid with H2/D2 increases the quantity of these gases in Au NPs. If the solidification rate of NPs is sufficiently high, then the dissolved gases remain inside the NPs.
Each nanoparticle can be considered as a target that is ionized by the laser pulse. The expansion of the plasma around the nanoparticle is confined by surrounding liquid, so that sufficiently long laser pulse can still interact with these nano-sized plasma entities.
Conclusion:
Further interpretation of the observed results on laser initiation of nuclear reactions cannot be performed on the basis of known phenomena. It seems that the gases dissolved in Au NPs provide the particles that further induce the nuclear reactions. The mechanism of the formation of these particles, most probably neutrons, remains unknown so far. However, the present results allow the conclusion that the energy spectrum of these neutrons depends on the number of experimental parameters, such as the nature of the aqueous environment, laser wavelength, peak power of laser radiation, etc. The mechanism of the initiation of nuclear reactions at relatively weak laser intensities of 10^13 W/cm^2 requires further multi-parametric studies.
Acknowledgements:
The work was partially supported by Russian Foundation for Basic Research, grants ## 07-02-00757, 08-07-91950, and by Scientific School 8108.2006.2. Dr. A.V. Goulynin is thanked for gamma-measurements and helpful discussions.
References:
1. A.V. Andreev, V.M. Gordienko, A.M. Dykhne, A.B. Saveliev, E.V. Tkalya, JETP Lett. 66 (5) (1997) 312
2. A.V. Andreev, R.V. Volkov, V.M. Gordienko, A.M. Dykhne, P.M. Mikheev, E.V. Tkalya, O.V. Chutko, A.A. Shashkov, JETP Lett. 69 (5)(1999) 343.
3. H. Schwoerer, P. Gibbon. S. Düsterer, R. Behrens, C. Ziener, C. Reich, R. Sauerbrey, Phys. Rev. Lett., 86 (11), (2001) 2317.
4. V.S. Belyaev, A.P. Matafonov, V.I. Vinogradov, V.P. Krainov, V.S. et al, Phys. Rev. E, 72 026406 (2005).
5. G. A. Shafeev, F. Bozon-Verduraz, and M. Robert, Physics of Wave Phenomena, 15(3) (2007) 131–136.
6. G.A. Shafeev, A.V. Simakin, F. Bozon-Verduraz, M. Robert, Appl. Surf. Sci., 254 (2007) 1022–1026.
7. A.V. Simakin, G.A. Shafeev, Physics of Wave Phenomena, 16 (4) (2008) 268-274.
8. G.A. Shafeev, Laser-based formation of nanoparticles, in: Lasers in Chemistry, Volume 2: Influencing matter. Edited by M. Lackner, Wiley VCH, Wienheim, pp. 713 – 741(2008).
9. A.V. Simakin, G.A. Shafeev, Initiation of nuclear reactions under laser irradiation of Au nanoparticles in the presence of Thorium aqua-ions, arXiv:0906.4268

Katheryn Mallette:
Thank you for your attention to our work,
Warm Regards,
A.R.
http://www.researchgate.net/publication/330601653/publication_E-Cat_SK_and_long_range_particle_interactions
Having read this I believed it was really enlightening. No surprise at all that with 21300 full readings and counting it is the nuclear physics paper most read in the world during the year on course.
Gosspeed,
Katherine
Abraham Herzog:
Obviously, the E-Cats will be available for anyone who will buy them, independently from her or his geographical, political, racial and/or religious belongings.
Warm Regards,
A.R.
Dr Andrea Rossi:
Your technology will be available in all the world or there will be political limitations?
Cheers,
Abraham
Dear Enrico T.:
Which statement?
Warm Regards,
A.R.
This is the precise weblog for anyone who wants to seek out out about this topic. You realize so much its nearly hard to argue with you (not that I truly would want…HaHa). You definitely put a brand new spin on a topic thats been written about for years. Nice stuff, just great!
Thank you for this web-site, all is excellent! Your Articles happen to be above and beyond other folks about this topic. Thank you so much for your help.
On the laser theme, we suggest to our Readers to read this interesting paper on Arxiv:
http://arxiv.org/abs/1009.0703
This paper is about the fact that in high energy conditions a single electron in interaction with a pulse of the e.m. field can produce cascades of e+, e-.
A.R.
Dear Jacques,
Of course I will be glad to meet you, but I am usually in the USA: anyway, I will be in Italy from the 6th through the 16th of January, and I will be glad to meet you. Anyway: probably I will have to be in Paris shortly, in this case I will come to meet you.
Warm Regards,
Andrea
I indeed think that the Rossi system could provide a way for carrying out such reactions, provided the conditions are well defined. This needs to be discussed in details. Andrea, it is possible I have some business to do in Italy during January(probably mid January, still to be defined). If so and if you are in Italy during this period, we could meet to discuss.
Warms regards.
Jacques
Before Copernicus it was well known that Sun orbited around the Earth. Since millennia.
A.R.
It is remarkable that the EM laser power interacting with the nanoparticles can induce these reactions. Usually in the text books it usually written the nuclear properties cann’t be affected by envirnment conditions, but strong variation of electromagnetic field generates induced nuclear reactions. So the system of Rossi-Focardi could be one particular case of such reactions.
Thanks for this post. I learned a lot from it. Hope to read more.